From antibiotics to cardiovascular to respiratory, most types of medicines continue to be in short supply. PGEU annual medicine shortages report indicates that in 2023 the situation grew worse compared to previous years. In the Netherlands alone 2.292 shortages were registered last year affecting about 5 million people. Other countries like Sweden, Portugal and Spain saw a significant increase in the number of shortages.
PGEU President Aris Prins said, “Despite pharmacists continued best efforts to find solutions, shortages still leave many patients without their prescribed treatment. This situation causes frustration and inconvenience for patients and erodes their trust in pharmacists and in the healthcare system. They also cause stress for pharmacy staff and impose an additional administrative burden on pharmacies daily work”.
In 2023 every pharmacy across the EU spent on average almost 10 hours per week dealing with medicine shortages. This time has tripled over the last 10 years; precious time which could be devoted to other useful tasks such as providing patients advice on the safe and effective use of medicines. Pharmacies struggle even more to provide patients solutions for shortages given the current healthcare workforce shortage.
Strong differences exist among countries regarding the options that pharmacists can explore to find alternatives – for example substitution or compounding – in case the prescribed medicine is unavailable. Pharmacists should be granted greater flexibility and enabled to leverage their skills, knowledge, and experience to efficiently assist patients.
“The reform of the EU pharmaceutical legislation is a unique opportunity to build a more resilient supply chain and improve shortages prevention, monitoring, and management. However, we need more immediate measures to address this chronic problem and reverse the negative trend that pharmacists have been denouncing for over a decade. We urge for earlier notification of shortages, for a timelier information to pharmacists and a fairer redistribution of medicines across countries” Prins added.
Each year PGEU conducts a survey among its members to map the impact of medicine shortages across Europe from the community pharmacists’ perspective. The 2023 Survey was open to all PGEU member organisations and has been conducted between 4 December 2023 and 17 January 2024. A total of 26 PGEU members responded to the survey, including Ireland.
Policy Recommendations
Community pharmacists are, in most cases, successful in minimising the negative impact of shortages on patients’ health and ensuring continuity of care. However, to effectively address the growing problem of medicine shortages in Europe, PGEU calls on policy makers to adopt urgent, bold, and ambitious measures, namely:
Ensure availability: Governments and stakeholders must put patients’ needs first when developing national laws and business strategies, respectively. These should first and foremost aim to ensure a timely and adequate supply of medicines to patients. These principles should apply also to the ongoing reform of the European pharmaceutical legislation, where patients’ interests should prevail over commercial ones. It is also necessary to ensure effective compliance with EU and national laws related to the public service obligations of supply chain actors. Policy makers also need to consider the impact of pricing policies on medicines availability and on the security of the supply chain.
Widen professional competence: The scope of pharmacy practice should be extended when medicines are in short supply, so pharmacists can use their skills, knowledge, and experience to better manage patient care and ensure continuity of treatment. When medicines are not available, pharmacists should be allowed to substitute with the most appropriate alternative, as part of a shared decision-making process with prescribers and patients and/ or in accordance with national protocols. Shared electronic communication tools between pharmacists and prescribers (e.g., shared electronic health records) can facilitate this process effectively and safely.
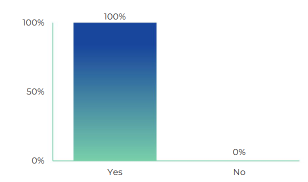
Question 1. In the last 12 months, have you experienced shortages of medicines in community pharmacy in your country?
(% of responding countries)
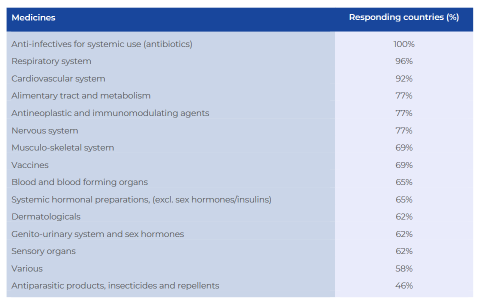
Question 3. If you have experienced shortages in the last 12 months in your country, which medicine classes have been in short supply in community pharmacy?
(ATC Level 1, multiple answers per country, % of responding countries)
Develop effective governance systems: A close collaboration between EU Member States and the European Medicines Agency (EMA) is needed to improve reporting, monitoring, and communication on medicine shortages. At national level, more structural, timely and transparent collaboration models between supply chain stakeholders and national competent authorities must be developed to increase the efficiency and effectiveness of joint notification and assessment practices, and to empower pharmacists in reducing the impact on European patients.
Improve communication: It is vital to ensure greater transparency and availability of medicine shortages data. Effective communication, early detection and central assessment of potential shortages can be achieved by connecting all medicine supply chain actors and NCAs at national level in consistent reporting systems. This will ensure that community pharmacists have timely information on current and foreseen medicine shortages. It is also necessary to increase access to the information available across the supply chain.
Compensate financial impact: The resource investment by pharmacists and pharmacies to manage shortages must be recognised and valued.
Survey Results
When asked if, in the last 12 months, have there been medicine shortages in community pharmacy in your country, 100% of responding countries (26) experienced medicine shortages in the last 12 months. Since 2019, all surveyed countries have experienced shortages.
Compared to the previous 12 months, in 2023, in 17 of the 26 responding countries the situation grew worse (65%) and stayed the same in 6 countries (23%). Only 3 countries, namely Cyprus, Greece and North Macedonia registered improvements when compared to the year before.
In 2022, 76% of the responding countries said the situation had gotten worse or stayed the same in 24% of the cases.
According to the Irish Pharmacy Union shortages have significantly increased in the last year and pharmacists in Ireland do not receive sufficient advance warnings of shortages.
69% of responding countries experienced shortages of medical devices in community pharmacy in the last 12 months, which shows a slight increase from last year’s situation (66%).
In terms of measuring how far medicine shortages have impacted on community pharmacies, over 90% said they had suffered financial loss due to time invested in mitigating shortages while over 80% said they had incurred increased administrative duties.
The Irish Pharmacy Union said the workload in management of shortages and associated phone calls from patients is rising year after year, leading to increased stress in pharmacy staff and mental health issues.
They added that there is an ongoing legislative process for a medicine shortage protocol that allows for therapeutic substitution by a pharmacist in limited circumstances where a medicine is in short supply. Normally a pharmacist liaises with the prescriber for an alternative medicine to be supplied, still requiring a new prescription.
In 12 out of the 26 responding countries (46%) there is a commonly agreed definition of medicine shortages at national level, and in 5 countries (19%) the definition of medicine shortages is enshrined in the national legislation.
In 18 out of the 26 countries surveyed (69%), there are reporting systems for shortages in place that can be used by community pharmacists.
Conclusion
In the last 12 months, all countries that responded to the PGEU survey experienced medicine shortages in community pharmacies. Similar to the previous year, a majority of these countries (65%) noted a deterioration in the situation compared to the preceding 12 months. In a few instances, the situation remained unchanged (23%), while a smaller percentage of countries reported positive improvements (12%).
Medicine shortages in community pharmacies have an impact on every medicine class across the countries surveyed – shortages of anti-infectives for systemic use (such as antibiotics) were experienced in all countries. Furthermore, 96% of the countries registered shortages of medicines for the respiratory and cardiovascular systems (92%). The most frequent medicines in shortage were anti-infectives for systemic use, medicines for the cardiovascular system, and medicines for the nervous system.
In a significant number of countries that participated in the survey (27%), the list of medicines facing shortages exceeded 600 at the time of survey completion. When compared to data from the previous year, both evidence and feedback from pharmacists indicate a worsening of the situation, with an increasing number of countries reporting a larger number of medicines in short supply over the course of the year.
A total of 69% of the countries that contributed to this year’s study reported encountering shortages of medical devices in community pharmacies, reflecting a slight increase from the previous year’s figure of 66%. These shortages span across all categories of medical devices. However, in only 2 countries there are systems to monitor shortages of medical devices, making it difficult to have a clear picture of the situation across Europe.
All responding countries indicated that they believe medicine shortages cause distress and inconvenience to patients. The most perceived consequences include treatment interruptions (reported by 88% of countries), increased co-payments due to more expensive or nonreimbursed alternatives (73%), and suboptimal treatment with reduced efficacy (73%). Notably, these percentages have shown an increase compared to the previous years.
Available solutions to community pharmacists in the event of shortages vary significantly across European countries. Generic substitution (92% of countries), preparing compounding formulations (50%), and adjusting therapy and posology when the same medicine is available in a different strength (50%) are commonly permitted solutions in many European countries. However, it is worth noting that some of these solutions may be subject to restrictions (e.g. requiring a new prescription). Additionally, the implementation of these solutions can be cumbersome and timeconsuming for both the patient and the pharmacist.











