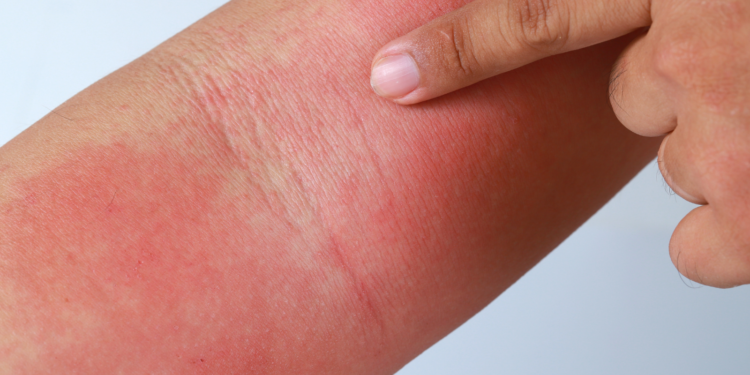
- Upadacitinib (30 mg, once daily) monotherapy demonstrated significantly greater rates of skin clearance improvement and itch reduction compared to dupilumab (300 mg, every other week) monotherapy at 16 weeks1
- Early, significant results were seen in upadacitinib-treated patients – including skin clearance improvements (EASI 75) at two weeks and itch reduction at one week – compared to dupilumab1
- The safety profile of upadacitinib was consistent with previous atopic dermatitis studies, with no new safety risks observed1-3
Dublin, Ireland 23rd September 2021: JAMA Dermatology has published 24-week results from the Phase 3b Heads Up study evaluating the efficacy and safety of upadacitinib (30 mg, once daily) versus dupilumab (300 mg, every other week) – both as monotherapy treatments – in adults with moderate to severe atopic dermatitis who were candidates for systemic therapy.
The publication showed upadacitinib (30 mg, once daily) achieved superiority compared to dupilumab for the primary endpoint, the proportion of patients with at least a 75 percent improvement in the Eczema Severity Index (EASI 75) at week 16.1 Of those treated with upadacitinib, 71 percent achieved EASI 75 at week 16 compared to 61 percent of those treated with dupilumab.1 Additionally, upadacitinib demonstrated statistically significant greater efficacy across all ranked secondary endpoints compared to dupilumab through week 16, including early reduction in itch and rates of skin clearance improvement.1
Professor Brian Kirby, Consultant Dermatologist at St Vincent’s University Hospital, who was an investigator in this study, said that “it was welcome that an Irish centre had the opportunity to participate in one of the first head-to-head Studies in Atopic Dermatitis. Not only does this study greatly add to our understanding of this disease and its treatment, but Irish patients got the benefit of access to treatments they would not have otherwise had.”
Results for select ranked secondary endpoints include:
- After one week of treatment, the upadacitinib 30 mg treatment group had a 31 percent reduction in itch (as measured by Worst Pruritus Numerical Rating Scale [NRS]) compared to 9 percent in the dupilumab group (p<0.001).1
- After two weeks of treatment, 44 percent receiving upadacitinib achieved EASI 75 versus 18 percent receiving dupilumab (p<0.001). 1
- At 16 weeks, 28 percent of people treated with upadacitinib achieved clear skin (EASI 100; p<0.001) and 61 percent achieved almost clear skin (EASI 90; p<0.001), compared to 8 percent and 39 percent, respectively, of those treated with dupilumab.1
The recommended dose of upadacitinib for Atopic Dermatitis is 15 mg or 30 mg once daily based on individual patient presentation.4
- A dose of 30 mg once daily may be appropriate for patients with high disease burden.4
- A dose of 30 mg once daily may be appropriate for patients with an inadequate response to 15 mg once daily.4
- The lowest effective dose for maintenance should be considered.4
- For patients ≥ 65 years of age, the recommended dose is 15 mg once daily.4
The safety profile of upadacitinib in Heads Up was consistent with what was observed in the Phase 3 pivotal studies, Measure Up 1, Measure Up 2 and AD Up.1-3 Through week 16, the most common adverse events were acne (15.8%) for the upadacitinib group and conjunctivitis (8.4%) for the dupilumab group.1 Serious adverse events occurred in 2.9 percent of those receiving upadacitinib and 1.2 percent of those receiving dupilumab.1 Serious infections were numerically higher in upadacitinib group, although at generally low levels (1.1 percent in those who received upadacitinib and 0.6 percent in those who received dupilumab).1 One treatment-emergent death due to bronchopneumonia associated with influenza A occurred in a patient who received upadacitinib.1 No malignancies were reported in the upadacitinib group; one non-melanoma skin cancer was reported in the dupilumab group.1 No major adverse cardiac events or venous thromboembolic events were reported in either treatment group.1 In the placebo-controlled atopic dermatitis clinical trials, the most commonly reported adverse reactions of patients with upadacitinib 15 mg or 30 mg were upper respiratory tract infection, acne, herpes simplex, headache, CPK increased, cough, folliculitis, abdominal pain, nausea, neutropenia, pyrexia, and influenza.1
About Heads Up1
Heads Up is a Phase 3b multicentre, randomised, double-blind, double-dummy, active comparator-controlled study in adults with moderate to severe atopic dermatitis. Patients were randomized to receive upadacitinib (30 mg, once daily, orally administered) or dupilumab (300 mg, every other week, subcutaneous injection) for 24 weeks. Patients who received dupilumab received an initial dose of 600 mg at the baseline visit followed by 300 mg every other week. All patients received placebo of the other treatment as part of the Heads Up double-dummy study design.
The primary endpoint was the proportion of patients achieving EASI 75 at week 16. Ranked secondary endpoints were percent change from baseline in Worst Pruritus NRS (weekly average) at weeks 1, 4 and 16; proportion of patients achieving EASI 100 and EASI 90 at week 16; proportion of patients achieving EASI 75 at week 2; and Worst Pruritus NRS (weekly average) improvement ≥4 at week 16. Additional endpoints at week 24 included EASI 75, EASI 90, EASI 100 and improvement from baseline Worst Pruritus NRS (weekly average). More information on this trial can be found at www.clinicaltrials.gov (NCT03738397).
About Upadacitinib
Discovered and developed by AbbVie scientists, upadacitinib is a selective and reversible JAK inhibitor that is being studied in several immune-mediated inflammatory diseases.4-15 In human cellular assays, upadacitinib preferentially inhibits signalling by JAK1 or JAK1/3 with functional selectivity over cytokine receptors that signal via pairs of JAK2.4 Upadacitinib 15 mg is approved by the European Commission for adults with moderate to severe active rheumatoid arthritis, adults with active psoriatic arthritis (PsA), adults with active ankylosing spondylitis (AS), and adults and adolescents 12 years and older with atopic dermatitis (AD).4
Phase 3 trials of upadacitinib in rheumatoid arthritis, atopic dermatitis, psoriatic arthritis, axial spondylarthritis, Crohn’s disease, ulcerative colitis, giant cell arteritis and Takayasu arteritis are ongoing.7-15
Important EU Indications and Safety Information about upadacitinib4
Rheumatoid arthritis
RINVOQ is indicated for the treatment of moderate to severe active rheumatoid arthritis in adult patients who have responded inadequately to, or who are intolerant to one or more disease-modifying anti-rheumatic drugs (DMARDs). RINVOQ may be used as monotherapy or in combination with methotrexate.
Psoriatic arthritis
RINVOQ is indicated for the treatment of active psoriatic arthritis in adult patients who have responded inadequately to, or who are intolerant to one or more DMARDs. RINVOQ may be used as monotherapy or in combination with methotrexate.
Ankylosing spondylitis
RINVOQ is indicated for the treatment of active ankylosing spondylitis in adult patients who have responded inadequately to conventional therapy.
Atopic dermatitis
RINVOQ is indicated for the treatment of moderate to severe atopic dermatitis in adults and adolescents 12 years and older who are candidates for systemic therapy.
Contraindications4
RINVOQ is contraindicated in patients hypersensitive to the active substance or to any of the excipients, in patients with active tuberculosis (TB) or active serious infections, in patients with severe hepatic impairment, and during pregnancy.
Special warnings and precautions for use4
Immunosuppressive medicinal products
Use in combination with other potent immunosuppressants is not recommended.
Serious infections
Serious and sometimes fatal infections have been reported in patients receiving upadacitinib. The most frequent serious infections reported included pneumonia and cellulitis. Cases of bacterial meningitis have been reported. Among opportunistic infections, TB, multidermatomal herpes zoster, oral/oesophageal candidiasis, and cryptococcosis have been reported with upadacitinib. As there is a higher incidence of infections in patients ≥65 years of age, caution should be used when treating this population.
Viral reactivation
Viral reactivation, including cases of herpes zoster, was reported in clinical studies. The risk of herpes zoster appears to be higher in Japanese patients treated with upadacitinib.
Vaccinations
The use of live, attenuated vaccines during or immediately prior to therapy is not recommended. It is recommended that patients be brought up to date with all immunizations, including prophylactic zoster vaccinations, prior to initiating upadacitinib, in agreement with current immunization guidelines.
Malignancy
The risk of malignancies, including lymphoma is increased in patients with rheumatoid arthritis (RA). Malignancies, including nonmelanoma skin cancer (NMSC), have been reported in patients treated with upadacitinib. Consider the risks and benefits of upadacitinib treatment prior to initiating therapy in patients with a known malignancy other than a successfully treated NMSC or when considering continuing upadacitinib therapy in patients who develop a malignancy.
Haematological abnormalities
Treatment should not be initiated, or should be temporarily interrupted, in patients with haematological abnormalities observed during routine patient management.
Cardiovascular risk
RA patients have an increased risk for cardiovascular disorders. Patients treated with upadacitinib should have risk factors (e.g., hypertension, hyperlipidaemia) managed as part of usual standard of care.
Lipids
Upadacitinib treatment was associated with dose-dependent increases in lipid parameters, including total cholesterol, low-density lipoprotein cholesterol, and high-density lipoprotein cholesterol.
Hepatic transaminase elevations
Treatment with upadacitinib was associated with an increased incidence of liver enzyme elevation compared to placebo.
Venous thromboembolisms
Events of deep vein thrombosis (DVT) and pulmonary embolism (PE) have been reported in patients receiving JAK inhibitors, including upadacitinib. Upadacitinib should be used with caution in patients at high risk for DVT/PE.
Adverse reactions4
The most commonly reported adverse reactions in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis clinical trials (≥2% of patients in at least one of the indications) with upadacitinib 15 mg were upper respiratory tract infections, blood creatine phosphokinase (CPK) increased, alanine transaminase increased, bronchitis, nausea, cough, aspartate transaminase increased, and hypercholesterolemia.
The most commonly reported adverse reactions in atopic dermatitis trials (≥2% of patients) with upadacitinib 15 mg or 30 mg were upper respiratory tract infection, acne, herpes simplex, headache, CPK increased, cough, folliculitis, abdominal pain, nausea, neutropenia, pyrexia, and influenza.
Ankylosing spondylitis:
Overall, the safety profile observed in patients with active ankylosing spondylitis treated with upadacitinib 15 mg was consistent with the safety profile observed in patients with RA.
Psoriatic arthritis:
Overall, the safety profile observed in patients with active psoriatic arthritis treated with upadacitinib 15 mg was consistent with the safety profile observed in patients with RA. A higher incidence of acne and bronchitis was observed in patients treated with upadacitinib 15 mg (1.3% and 3.9%, respectively) compared to placebo (0.3% and 2.7%, respectively). A higher rate of serious infections (2.6 events per 100 patient-years and 1.3 events per 100 patient-years, respectively) and hepatic transaminase elevations (ALT elevations Grade 3 and higher rates 1.4% and 0.4%, respectively) was observed in patients treated with upadacitinib in combination with MTX therapy compared to patients treated with monotherapy. There was a higher rate of serious infections in patients ≥65 years of age, although data are limited.
Atopic dermatitis:
Dose-dependent changes in ALT increased and/or AST increased (≥ 3 x ULN), lipid parameters, CPK values (> 5 x ULN), and neutropenia (ANC < 1 x 109 cells/L) associated with upadacitinib treatment were similar to what was observed in the rheumatologic disease clinical studies. Based on limited data in atopic dermatitis patients aged 65 years and older, there was a higher rate of overall adverse reactions with the upadacitinib 30 mg dose compared to the 15 mg dose. The safety profile for upadacitinib 15 mg in adolescents was similar to that in adults. The safety and efficacy of the 30 mg dose in adolescents are still being investigated.
This is not a complete summary of all safety information.
Please see the RINVOQ full SmPC for complete prescribing information at www.medicines.ie
About AbbVie
AbbVie’s mission is to discover and deliver innovative medicines that solve serious health issues today and address the medical challenges of tomorrow. We strive to have a remarkable impact on people’s lives across several key therapeutic areas: immunology, oncology, neuroscience, eye care, virology, women’s health, and gastroenterology, in addition to products and services across its Allergan Aesthetics portfolio. For more information about AbbVie, please visit us at www.abbvie.com. Follow @abbvie on Twitter, Facebook, LinkedIn, or Instagram.
Forward-Looking Statements
Some statements in this news release are, or may be considered, forward-looking statements for purposes of the Private Securities Litigation Reform Act of 1995. The words “believe,” “expect,” “anticipate,” “project” and similar expressions, among others, generally identify forward-looking statements. AbbVie cautions that these forward-looking statements are subject to risks and uncertainties that may cause actual results to differ materially from those indicated in the forward-looking statements. Such risks and uncertainties include, but are not limited to, failure to realize the expected benefits from AbbVie’s acquisition of Allergan plc (“Allergan”), failure to promptly and effectively integrate Allergan’s businesses, competition from other products, challenges to intellectual property, difficulties inherent in the research and development process, adverse litigation or government action, changes to laws and regulations applicable to our industry and the impact of public health outbreaks, epidemics or pandemics, such as COVID-19. Additional information about the economic, competitive, governmental, technological and other factors that may affect AbbVie’s operations is set forth in Item 1A, “Risk Factors,” of AbbVie’s 2020 Annual Report on Form 10-K, which has been filed with the Securities and Exchange Commission, as updated by its subsequent Quarterly Reports on Form 10-Q. AbbVie undertakes no obligation to release publicly any revisions to forward-looking statements as a result of subsequent events or developments, except as required by law.
References:
- Blauvelt, A., et. al. A Phase 3 Trial of Upadacitinib Versus Dupilumab in Atopic Dermatitis. JAMA Dermatology doi:10.1001/jamadermatol.2021.3023
- Guttman-Yassky E., et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate, double-blind, randomized controlled phase 3 studies. Lancet. doi:10.1016/s0140-6736(21)00588-2.
- Reich K., et al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD Up): results from a randomized, double-blind, placebo-controlled phase 3 trial. Lancet. doi:10.1016/s0140-6736(21)00589-4.
- RINVOQ [Summary of Product Characteristics]. AbbVie; August 2021. Available at:medicines.ie
- Cohen S., et al. Safety profile of upadacitinib in rheumatoid arthritis: integrated analysis from the SELECT phase III clinical programme. Ann Rheum Dis. 2020 Oct 28;80(3):304-11.
- Mease, P.J., et al. Upadacitinib in Patients with Psoriatic Arthritis and Inadequate Response to Biologics: 56-Week Data from the Randomized Controlled Phase 3 SELECT-PsA 2 Study. Rheumatol Ther. 2021 Apr 28. doi: 10.1007/s40744-021-00305-z. Online ahead of print.
- Pipeline – Our Science | AbbVie. AbbVie. 2019. Available at: https://www.abbvie.com/our-science/pipeline.html. Accessed on August 17, 2020.
- A Study to Evaluate Efficacy and Safety of Upadacitinib in Adult Participants With Axial Spondyloarthritis (SELECT AXIS 2). ClinicalTrials.gov. 2021. Available at: https://clinicaltrials.gov/ct2/show/NCT04169373. Accessed on July 27, 2021.
- Evaluation of Upadacitinib in Adolescent and Adult Patients With Moderate to Severe Atopic Dermatitis (Eczema) (Measure Up 1). ClinicalTrials.gov. 2021. Available at: https://clinicaltrials.gov/ct2/show/ NCT03569293. Accessed on July 27, 2021.
- A Study of the Efficacy and Safety of Upadacitinib (ABT-494) in Participants With Moderately to Severely Active Crohn’s Disease Who Have Inadequately Responded to or Are Intolerant to Biologic Therapy. ClinicalTrials.gov. 2021. Available at: https://clinicaltrials.gov/ct2/show/NCT03345836. Accessed on Accessed on July 27, 2021.
- A Study to Evaluate the Safety and Efficacy of Upadacitinib (ABT-494) for Induction and Maintenance Therapy in Participants With Moderately to Severely Active Ulcerative Colitis (UC). ClinicalTrials.gov. 2021. Available at: https://clinicaltrials.gov/ct2/show/NCT02819635. Accessed on July 27, 2021.
- A Study Comparing Upadacitinib (ABT-494) to Placebo in Adults With Rheumatoid Arthritis on a Stable Dose of Conventional Synthetic Disease-Modifying Antirheumatic Drugs (csDMARDs) Who Have an Inadequate Response to csDMARDs Alone (SELECT-NEXT). ClinicalTrials.gov. 2021. Available at: https://clinicaltrials.gov/ct2/show/NCT02675426. Accessed on July 27, 2021.
- A Study Comparing Upadacitinib (ABT-494) to Placebo and to Adalimumab in Participants With Psoriatic Arthritis Who Have an Inadequate Response to at Least One Non-Biologic Disease Modifying Anti-Rheumatic Drug (SELECT – PsA 1). ClinicalTrials.gov. 2020. Available at: https://clinicaltrials.gov/ct2/show/NCT03104400. Accessed on August 17, 2020
- A Study to Evaluate the Safety and Efficacy of Upadacitinib in Participants With Giant Cell Arteritis (SELECT-GCA). ClinicalTrials.gov. 2021. Available at: https://clinicaltrials.gov/ct2/show/NCT03725202. Accessed on July 27, 2021.
- A Study to Evaluate the Efficacy and Safety of Upadacitinib in Subjects With Takayasu Arteritis (TAK) (SELECT-TAK). ClinicalTrials.gov. 2021. Available at: https://clinicaltrials.gov/ct2/show/NCT04161898. Accessed on July 27, 2021.