Introduction
In disease control, prevention is superior, safer, and generally more cost-effective than treating illnesses after they develop. Vaccination, which is the administration of antigenic material to produce immunity to a disease, is one of the most important preventive measures currently at our disposal. The immune response to a vaccine may be cell-mediated or antibodymediated, or both. Human papillomaviruses (HPVs) comprise a group of about 100 DNA nonenveloped viruses. The burden of disease related to HPV is substantial.
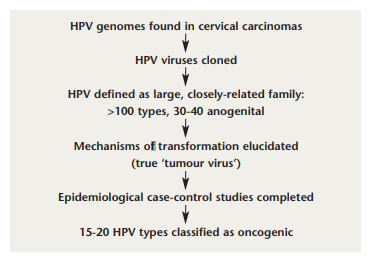
FIGURE 1: Discovery of the link between human papillomavirus and cervical cancer. Adapted from: Pichichero ME. Prevention of cervical cancer through vaccination of adolescents. Clin Pediatr (Phila) 2006; 45 (5): 393-8.
Variable proportions of penile,1 vaginal,2 urethral,3 and head and neck cancers have been found to contain carcinogenic HPV.4-6 Cervical cancer is the most important manifestation of genital HPV infection, and is one of the leading causes of cancer mortality in women worldwide. The global disease burden of cervical cancer is estimated at 470,000 new cases, and 230,000 deaths, every year. Almost 80% of cases occur in developing countries, where it is the most common cancer among women.7 In Europe, 15,000 women die from cervical cancer each year, and it is the second most common form of cancer seen in women under 35 years of age. In Ireland, there are approximately 180 new cases annually, and about 80 deaths a year are caused by cervical cancer.8 Cervical cancer primarily affects women between 39 and 45 years of age, thereby representing an important opportunity cost in terms of potential years of life lost. The impact of the disease is accentuated further by the average age at death, often when women are still raising children. In the United States, it is estimated that over six million people are infected with genital HPV.9 Data from the National Health and Nutrition Examination Survey found a 26.8% prevalence of HPV among US girls and women, with increasing prevalence each year from ages 14 to 24 years, followed by a gradual decline in prevalence until 60.10 The risk factors for acquisition of HPV infection are shown in Table 1. Infection is extremely common in young women in their first decade of sexual activity.11 Condoms afford some protection against HPV transmission, although this protection is incomplete.5

Taken from: Pichichero ME. Prevention of cervical cancer through vaccination of adolescents. Clin Pediatr (Phila) 2006; 45 (5): 393-8.
The World Health Organisation commented recently that a vaccine to prevent oncogenic HPV infection or premalignant cervical lesions from progressing to cancer would offer a cost effective long-term strategy to reduce the cervical cancer burden, particularly for developing countries where effective screening programmes are not available.12 The aim of this review was to examine the evidence for and against vaccination of children and teenagers against HPV and the development of cervical cancer.12
Methods
A MEDLINE and PubMed search (1980-2007) was performed using the search terms ‘HPV vaccination’, ‘HPV vaccination and children’, ‘Cervical cancer and HPV’, and ‘CIN’. An English language restriction was imposed and the search continued up to August 20, 2007. The references cited in review articles were examined for potentially eligible studies. The literature search yielded 160 papers, of which 39 were considered eligible for inclusion.
Results
HPV and cervical cancer. Key landmarks in the discovery of the link between HPV and cervical cancer are illustrated in Figure 1. The first recognition of this relationship was made by Harald zur Hausen in the 1970s.13 Using sensitive molecular polymerase chain reaction (PCR) techniques, Walboomers et al detected HPV DNA in 99.7% of a series of cervical cancers.14 More than 100 strains of HPV exist, but types 16 and 18 are the most prevalent oncogenic strains of the virus, with HPV 16 accounting for more than 60% of cervical cancers, and HPV 18 responsible for another 10%.15-18 The great majority of HPV infections, including infection by carcinogenic types, are transient, and resolve or become undetectable within a year or two, sometimes causing mild cytopathological changes, including atypical squamous cells (ASC), low-grade squamous intraepithelial lesions (LSIL) and Grade 1 cervical intraepithelial neoplasia (CIN) changes.19-22 Most women will get oncogenic HPV at some stage in their lives, usually in their teens or twenties, and it will pass harmlessly. A very small proportion persists, and becomes integrated into the cell DNA. These pose a risk of cancer to the woman in question. About 500,000 precancerous lesions of Grade 2 and 3 (CIN 2 and 3) are diagnosed each year in the United States, and about 50-60% are attributable to HPV 16 and HPV 18.23
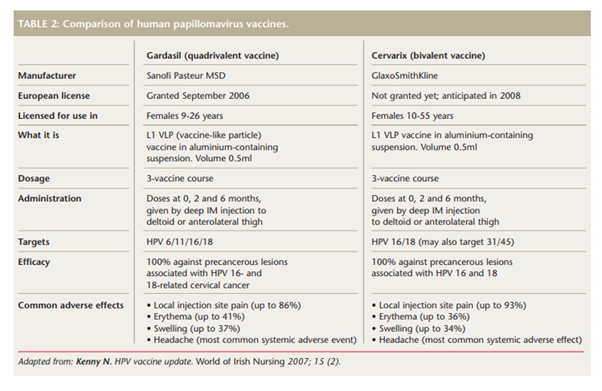
Adapted from: Kenny N. HPV vaccine update. World of Irish Nursing 2007; 15 (2).
HPV vaccines
Two vaccines have been developed to prevent HPV infection and details of these are shown in Table 2. Randomised controlled trials of HPV vaccines have demonstrated their immunogenicity and ability to prevent incident and persistent type-specific HPV infection and associated cytological abnormalities in the cervix.24 Intramuscular injection of either vaccine induces high titres of neutralising antibody, at more than 50 times the titres induced by natural infection.25 Phase II trial results for both vaccines have shown efficacy estimates of 100% against either incident or persistent HPV infection.26
In a seminal randomised, double blind, controlled trial to assess the efficacy, safety, and immunogenicity of a bivalent HPV 16/18 L1 virus-like particle vaccine (similar to Cervarix), Harper et al randomised 1,113 women aged between 15 and 25 years. These women received three doses of either the vaccine or placebo on a 0-, 1-, and 6-month schedule in North America and Brazil. Women were assessed for HPV infection for up to 27 months by cervical cytology and self-obtained cervicovaginal samples, and for vaccine safety and immunogenicity. The vaccine efficacy against incident infection was 91.6% (95% CI, 64.5–98.0) and 100% against persistent infection (95% CI, 47.0–100), as well as also protecting against HPV-associated cytological abnormalities and lesions.27 In a follow-up study in 2006, Harper et al found that more than 98% of subjects who received all three doses remained seropositive for HPV 16/18.28 While it is true that vaccination may not have an effect on cervical cancer for 20 to 30 years, vaccination could profoundly reduce the risk of CIN in about 10 years.
Two major randomised controlled trials – the FUTURE (Females United to Unilaterally Reduce Endo/Ectocervical Disease) II trial29 and the PATRICIA (PApilloma TRIal against Cancer In young Adults) study30 – were included in a systematic review by Rambout et al 31 This systematic review found that HPV vaccination was highly effective in preventing vaccine type-specific HPV infection, and precancerous cervical disease, particularly among women aged 15 to 25 years who received all three vaccine doses, had no more than six lifetime sexual partners, and no prior abnormal results from cervical screening. The FUTURE II study found that in young women who had not previously been infected with HPV 16 or HPV 18, those in the vaccine group had a significantly lower occurrence of high-grade CIN related to HPV 16 or HPV 18 compared with the placebo group.29 Both trials demonstrated high efficacy against CIN 2 or worse related to HPV types 16 and 18, with a mean follow-up of three years (FUTURE II) and 15 months (PATRICIA), respectively.31
Discussion
It is important to vaccinate patients before the age at which exposure to HPV is likely to occur, and from a public health perspective, routine vaccination before sexual debut or shortly thereafter is important. Recent Irish data found that 12% of women had their first experience of vaginal intercourse before the age of 17.8 years,32 and a poll of 15- to 17-year-olds found that one in four had had sex.33 The 2007 guidelines from the American Cancer Society recommend routine HPV vaccination for females aged 11 to 12 years but also state that females as young as nine years may receive the vaccine.34
In a population-based study to assess parental consent and potential HPV vaccine uptake, Brabin et al used a questionnaire to randomly sample parents of pupils aged 11 to 12 years in seven different secondary schools in the city of Manchester.35 They found a HPV vaccine uptake rate of 80% was achievable if the vaccine was perceived as safe and effective. However, the authors also found that most parents lacked knowledge about HPV, and some were concerned about sexual health issues that would arise as part of a HPV vaccine programme, as well as the possibility that administering the vaccine might actually encourage promiscuity.35
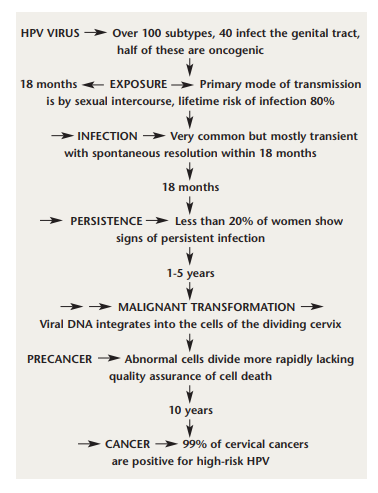
Cost-effectiveness studies of HPV vaccination have had variable results, depending on assumptions about effectiveness and safety.36 The major financial benefit of the vaccine to the Irish health service is likely to be the prevention of anogenital warts that the quadrivalent virus affords. Anogenital warts represent about 10% of sexually-transmitted HPV infections and 4,147 cases of anogenital warts were notified in Ireland in 2004.8 A research group at Stanford University found that if all 12-year-old girls currently living in the United States were vaccinated, more than 1,300 deaths from cervical cancer during their lifetimes would be averted,37 but as the United States does not have a national cervical screening programme, their cost-effectiveness calculations may be skewed.
The estimated cost of the quadrivalent HPV vaccine is $360 in the USA, and ¤450 in Europe for a three-course series, making it among the most expensive of all vaccines.38 If consumers or insurers were to pay, poor and uninsured persons would be unable to afford the vaccine, further exacerbating health disparities. If the government is to pay, this will require funds from the central budget, perhaps reducing health spending for other programmes.
In the wake of concerns about measles-mumps-rubella (MMR) vaccines, parents have become more cautious about vaccine safety and some parents are less trusting of scientific evidence.39,40 There are scant data on the duration of HPV vaccine induced immunity. Following naturally occurring HPV infection, many women do not develop detectable HPV antibodies and, in the case of HPV 16, the available serological assays detect type-specific antibodies in only 54-60% of infected women.20 Therefore, longer-term follow-up of vaccinated subjects cannot rely on routine measurement of HPV vaccine-induced antibody titres. Furthermore, because of the extended time involved in the progression from HPV infection to cervical cancer, it will be many years before a reduction in cancer incidence and mortality rates can be verified within a vaccinated population. The Canadian federal government recently announced a $300 million investment in a programme for vaccinating girls and women with the currently available Gardasil vaccine. Questions have been raised, however, as to whether a universal immunisation programme is, at this time, premature and could have unintended negative consequences for individuals and for society as a whole.41 A repeated comment is that there is no epidemic of cervical cancer in Canada to warrant the sense of urgency for a vaccination programme.
According to the 2006 Canadian cancer statistics,42 cervical cancer is the 11th most frequent cancer affecting women, and the 13th most common cause of cancerrelated deaths, accounting for approximately 400 deaths per year. Earlier this year the states of Texas (by executive order) and Virginia made quadrivalent HPV vaccine mandatory for girls entering sixth grade.43 However, the Texas legislature recently voted to overturn the governor’s order, and the state of Virginia granted parents generous “opt-out” provisions.44 Nearly 20 additional states are considering similar legislation.45 Research in the US on the issue of mandatory vaccination concluded that making the HPV vaccine mandatory might paradoxically contribute to long-standing parental concerns about the safety of school-based vaccination, and increase parental and public apprehension about the vaccine.46
Australia already has a government-funded programme offering vaccination to girls and young women aged 12 to 26 years.47 The United Kingdom has just announced that universal HPV vaccination of girls aged 12 to 13 years will begin in September 2008, and the UK Heath Secretary Alan Johnson has confirmed that the cervical screening programme will continue after the introduction of the HPV vaccine. It is expected that they will use the quadrivalent vaccine Gardasil.48
In Ireland, the Department of Health and Children has not yet introduced routine cervical cancer vaccination, or even a systematic screening programme.
In January 2006, the Minister for Health and Children, Mary Harney, announced the rollout of the Irish Cervical Screening Programme (ICSP). In parallel with the ICSP, she announced the establishment of the Board of the National Cancer Screening Service and, in a press release, stated that: “issues to be examined included the effectiveness and cost-effectiveness of the vaccine, categories of women who should be vaccinated, immunity duration and booster requirements, and the implications for the national screening programme”.49 In a properly screened programme, the vaccination of young girls will prevent CIN and colposcopy, as well as prevent cervical cancer. Vaccination provides a new approach to preventing cervical cancer in Ireland and in other countries with a high incidence of the disease and zero or poorly developed screening programmes.47 It is conceivable that if a vaccination programme were introduced in the absence of a screening programme, the rate of cervical cancer could actually increase. This message is reiterated by Adams et al, who stress the need to implement or continue a cervical screening programme to complement vaccination, which will optimise prevention in those who receive the vaccine and prevent cervical cancer in older women where the value of vaccination is currently unclear.50 Vaccination will reduce the risk of cervical cancer by another 20%.37
Conclusion
HPV vaccination has high efficacy against the major HPV types that cause life-threatening disease. Vaccination appears to be safe, and so delaying vaccination may mean that many women will miss an opportunity for long-lasting protection against cervical cancer. On the other hand, a cautious approach may be warranted in the light of important unanswered questions about overall vaccine efficacy, duration of protection and possible adverse effects.50 The advent of HPV vaccines represents the most important breakthrough to date in cervical cancer prevention, and will have positive consequences for public health worldwide.
Acknowledgements
I would like to thank my family for their encouragement to write this paper, and Professors Malone and Prendeville for their helpful comments.









